Immunotherapy drugs for breast cancer
Comments
-
Heidi - Wow. I sure wish I had access to your clinic. Oncology in the US has more in common with McDonalds. Even at the Mayo Clinic, supposedly the best hospital in the country. I may not understand the distinctions between different types of private clinics in Europe and their accreditation.
>Z<
0 -
Heidihill, I didn't realize you are here in Europe, as I am.
May I ask which clinic you're treated in? If you prefer not to share, I understand. And if it's in previous messages, I'll look back!I began following this conversation some time in early 2016.
Thanks to you all for the very informative conversation. This is what we need more of.
M
0 -
I had my fifth injection for the Tapimmune folate receptor trial yesterday. I have one more to go. This injection caused a larger reaction at the injection site. Immediately after the injection there was a raised, pinkish swelling about the size of a nickel. This has happened every month but usually that recedes after 2 hours. Last month it receded but came back larger, 1.5 x 1.5", about 12 hours later. This month it did not recede and increased in size over night. This morning it was 2.5 by 3.5 inches, pinkish & warm. Also itchy. It has held steady at that point. I don't know whether this is consistent to what others in the trial are experiencing. I still con't consider this a particularly bothersome side effect compared to what happened with ACT.
0 -
aterry - thanks for the update. do they do any testing to measure an immune response, like whether the Tcells are seeing the antigens and responding?
>Z<
0 -
Hi Z, Last month they took about a dozen vials of blood and I'm sure they were checking for all kinds of things, including T cells. Patients don't get any feedback about trial related results (at least not, yet). Some of the blood is tested for standard stuff that we had during chemo, like white blood cells, red blood cells, hemoglobin, etc. That panel I see the results for and I've been in the normal range for every item. Another test they do every month, which I did not have during chemo, is a urinalysis for protein. If protein is too high you can't have the injection. I don't know why. Mine has always been fine. The Tapimmune web site says that there will be preliminary results from this phase by mid 2018. I'd be curious to know what others have been experiencing. I think the majority of participants have finished.
0 -
have you had any replies? do you have an update on IO? hope all is well, thank you!
0 -
I had an email from the coordinator for the Tapimmune trial at the Dubin center. She said that my reaction (raised, deep pink swelling at injection site) to the 5th injection is similar to what a few other patients have experienced. She didn't elaborate. My 6th & last injection is scheduled for March 28th and I'll ask a lot of questions then.
0 -
yes, where are the immunotherapy clinical trials for BC ER + PR+, HER- ??
I'm in Texas. Know any good doctors incorporating building the immune system. and using acupuncture for pain instead of all the addicting opioids.
Diagnosed : Late Sept 2017, BC, ER+,PR+,HER-, stage 4 . Tumor on disc and strangle hold around spine. 4spots on liver. Grade 2. lymphedema both arms. none removed.
10 sessions radiation, 4mo IV chemo taxol. Grew cancer from .9 to 1.9 and 12 spots breaking thru skin!
Now Lipron shot to block Eggs, LETROZOLE to block hormone and IBRANCE.
Any other suggestions/ options?
Know any support groups in North Dallas area?
~Enjoy Life Now~
0 -
EnjoyLifeNow,
Did you try: www.BreastCancerTrials.org for immunotherapy? You can also look at: https://clinicaltrials.gov/. I also got a second opinion at Dana Farber (I'm in the New England area), but if I were in Texas (where I lived for 9 yrs) I would go to MDAnderson. I was very glad I went, my local oncologist encouraged me to do this because of the large # of clinical trials available there.
0 -
EnjoyLife - There are fewer immunotherapy trials for ERPR+ because it is harder to get the immune system to see ERPR+ cancer. Immunotherapy is in development so they want to prove it works on easier targets. Nonetheless, there are many many trials that accept ERPR+ patient. You have to search for solid tumors on clinicaltrials.gov rather than just breast cancer. They accept ERPR+ cancer in trials that target solid tumors generally.
>Z<
0 -
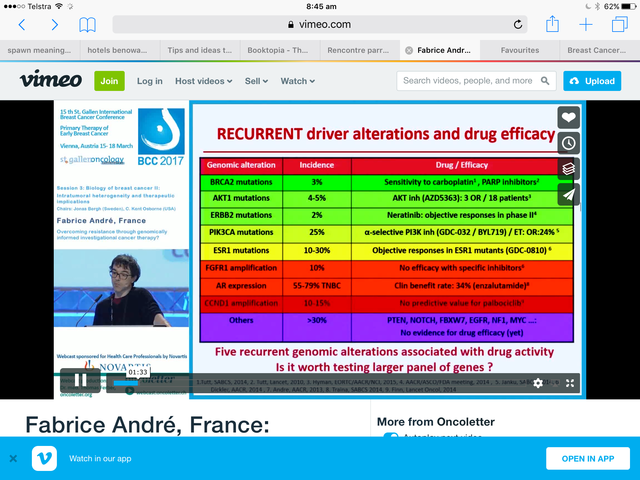
Fabrice André was mentioned by Cure-ious in Ibrance.
This is a talk I like - I can not post the Vimeo link but it's easy to find.
0 -
Wildplaced - thanks. Will look at that video today.
All - This is a list of immunotherapy trials for ERPR+ cancer that I am monitoring and evaluating. It's not edited or prioritized, or complete. It doesn't include oncolytics or vaccine trials (also immunotherapy), which are a separate list in my system. These are immune modulating drug trials that will accept ERPR+ cancer patients.
We have more than enough rope to hang ourselves, as they say, as far as immunotherapy trials go. Most of these trials are risky, all are early stage and without proven efficacy on ERPR+ cancer. Not advocating these trials, just answering EnjoyLife's question ... where are the immunotherapy trials for ERPR+ cancer.
T-Cell Therapy for Advanced Breast Cancer - Full Text View - ClinicalTrials.gov
Phase 1 Trial of Hu5F9-G4, a CD47-targeting Antibody - Full Text View - ClinicalTrials.gov
Palbociclib After CDK and Endocrine Therapy (PACE) - Full Text View - ClinicalTrials.gov
>Z<
0 -
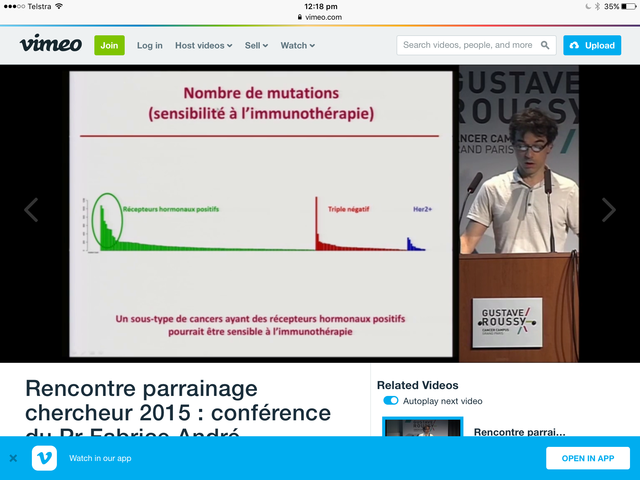
He keeps things deceptively simple - it's what I like about him.
This slide
from a talk at Gustave Roussy - it's in French. He is clear as of 2015 that there is a subgroup of ER + that is responsive to immunotherapy and that subgroup needs to be defined genomically.
In the States there is a man called Dejan Juric whose published work I like.
0 -
Thanks Wildplaces. I can't read the Y axis on the graph, and i can't google the talk ... what is the name of the video?
>Z<
0 -
Google:
Rencontre parrainage chercheur 2015: conference du Dr Fabrice André
I can't either but from the talk it seems to be the mutational loadand he implies that correlates for ER +ve with " Antigen du tumour".
0 -
My mutational load is 7 so I am wondering if I fit into the group he would consider responsive to immunotherapy. However, Dr. Andre is very focused on what is approved so immunotherapy = checkpoint inhibition. We do not know whether the mutational load drives other immune therapies like vaccines or adoptive cell therapy or oncolytics or other immune modulating drugs in development.
Z
0 -
Z
You won't know what he thinks unless you talk face to face. His email is probably not different from Romaine / the guy we wrote to on yoghurt and all. It's pretty standard for Paris Hospitals.
fabrice.andre@gustaveroussy.fr ( I think the e has the apostrophe)
You have done a lot of interesting stuff - the Japanese are always astrong side group - he might just answer.
☺️
0 -
Z,
You are right we do not know. But sometimes in the mist of names and titles and endless abbreviations one has to remember if it all makes sense. The universe is harmonious. Ha ! My uneducated guess, and I know shit about immunology is it probably does - we just do not know how to manipulated to get it to respond timely to high mutational loads
The historical fact that it took genetics to bring back us to immunotherapy in oncology is absurd. If common sense prevailed it would have been the other way around.
0 -
amen.
>Z<
0 -
Z and Wildplaces, you have so much information, how do you absorb it? I can only read about research in small doses or I can't understand it. I admire your work and effort.
0 -
This is no news, news. I had my 30 day follow up after my last injection on the Tapimmune trial. My MO did a clinical exam but there is no news about the outcome of the trial. The clinical trials coordinator at the Dubin Breast Center usually sits in on my appointments but she had a schedule conflict. This phase of the trial was to determine a dosing regimen. They were testing 4 variations on dosing. I had low dose with Cytoxan priming. Presumably they'll use data from this phase to choose which dose the participants in the next phase will receive. That phase is starting soon, I think. At Mayo, I think, with a larger cohort; 300, maybe?
0 -
aterry - Thanks for the update. There is a lot of information in the non-news that you report. For example, the fact that they are expanding to a large cohort is important and positive. Watching this closely. Please keep us apprised. Is there a point where you can evaluate the results/outcomes in your treatment?
>Z<
0 -
Zarovka, I don't think we'll get results based on our individual participation. But there will be cohort results. I'll get a 6 month booster injection. At that point it will be interesting to see whether I get the same injection--low dose--or whether they've chose high dose for the next phase and then I'll get that. They do a clinical breast exam each time and so far they have not detected anything to worry about. Other than that I think it's the same monitoring that we all get normally--the annual mammogram and/or ultrasound. I check the Tapimmune website every now and then just to see whether they've posted any updates but they haven't posted anything since late March.
0 -
thanks. very interested in anything you learn.
>Z<
0 -
So, here's some news out of ASCO ... NKTR-1 is a more tolerable form of Interluken-2 that clearly improves the performance of PDL-1 inhibitors. Interluken is nasty stuff but it looks like the found a more tolerable and possibly more effective form ...
Aldesleukin, recombinant human IL2, is an effective immunotherapy for metastatic melanoma and renal cancer, with durable responses in approximately 10% of patients; however, severe side effects limit maximal dosing and thus the number of patients able to receive treatment and potential cure. NKTR-214 is a prodrug of conjugated IL2, retaining the same amino acid sequence as aldesleukin.
Very early results, and not in ERPR+ MBC but ...
In a group of 13 melanoma patients, treatment with NKTR-214 and Opdivo demonstrated a impressive tumor response rate of 85 percent. That means 11 of the 13 patients responded.
The companies enrolled another 15 melanoma patients, and the response rate dropped. In the update, 14 of the now 28 treated patients (50 percent) showed significant tumor shrinkage. That means only three of the 15 added patients responded; however, the patients enrolled later in the study have not been followed very long. When they are, the companies expect more tumors to shrink, boosting the response rates.
The study presented at ASCO on Saturday night also included a group of patients with kidney cancer. Here, the initial response rate was 64 percent. Seven of 11 patients responded. But then 15 more kidney cancer patients were enrolled and the response rate dropped to 46 percent. Bristol and Nektar expect tumor responses are expected to get better over time.
The companies also pointed to data presented Saturday showing NKTR-214 may trigger tumor responses in so-called PD-L1 negative patients who don't typically respond to Opdivo alone. Five of 12 melanoma patients with PD-L1 negative tumors (42 percent) responded to the combination of NKTR-214 and Opdivo. For the 17 PD-L1 negative kidney cancer patients, the response rate was 53 percent.
0 -
I don't know if people have seen this and am not sure if this is the right place to post, but this woman's stage 4 cancer was cleared by her own immune system in a ground breaking treatment as reported by the Guardian (UK). https://www.theguardian.com/science/2018/jun/04/do...
Here is the lede and some introductory information from the article:
"A woman with advanced breast cancer which had spread around her body has been completely cleared of the disease by a groundbreaking therapy that harnessed the power of her immune system to fight the tumours.
It is the first time that a patient with late-stage breast cancer has been successfully treated by a form of immunotherapy that uses the patient's own immune cells to find and destroy cancer cells that have formed in the body.
Judy Perkins, an engineer from Florida, was 49 when she was selected for the radical new therapy after several rounds of routine chemotherapy failed to stop a tumour in her right breast from growing and spreading to her liver and other areas. At the time, she was given three years to live.
Doctors who cared for the woman at the US National Cancer Institute in Maryland said Perkins's response had been "remarkable": the therapy wiped out cancer cells so effectively that she has now been free of the disease for two years..."
Pub. Monday June 4, 2018
0 -
And, she is hormone receptor positive. Here's the abstract to the original article:
https://www.nature.com/articles/s41591-018-0040-8
This next article goes on to explain that it won't work for everyone, but still... this is good.
0 -
I know Judy. She posts on Inspire. Her miraculous treatment was a while a go and she is still off treatment and in remission.
She did the Tumor Infiltrating Lymphocyte trial. It's a tough trial. Not everyone who has done it has responded. Early on they had at least a couple deaths. I have not heard of any more deaths since they tweaked the protocol and became more selective in who they take. But not a slam dunk or a walk in the park.
Judy was also a very special case... there was a match between a target her cancer and a certain bullet they had just developed in the lab. I have heard the circumstances of her case described as winning the lottery.
>Z<
0 -
Wow, that is absolutely incredible and so inspiring! Can a cure be just around the corner?
0 -
it actually depresses me that Judy is the best the NIH can come up with for immunotherapy news. They dredging up very old (>2yrs) because thet need something for ASCO. They dont report any other MBC responders so that disturbs me. But at least they are talking about it... suggests they are still trying to figure it out.
Cure around the corner... Not from TILs.
Z
0