Are you currently (or have you been) in a Clinical Trial?
Comments
-
Dear CBL, I am with you - sometimes we also have to listen to what our 6th sense says, and, oh boy, how many times that 6th sense was right for me! Many hugs, Saulius
0 -
Thanks, everyone. I was glad for the experience and would do it again under different circumstances for sure. I have my fingers crossed for Orserdu!
Saulius, yes, I hope my anxiety didn't overrule my brain too much on this one—12 weeks is just too long for me to wait for scans. Maybe I'll be braver next time!
CBL
0 -
CBL, How great you did with all of this- perhaps it was on the bleeding edge of holding the cancer at check, and now Orserdu will easily kick it to the curb!!! This will be a very interesting drug to follow in future, these precision drugs with no side effects are wonderful.
1 -
CBL, What dose were you getting in the TTX-MC138 trial? They just announced the next (4th) group of patients, who will be getting 50% more drug, and note that some patients are still on the drug from prior cohorts (tho it hasn't been all that long) and that they think they are in therapeutic range, so yeah, maybe it was working to some extent which is wonderful!
0 -
I was on 3.24. I really hope this drug works out for everyone and I’ll feel like a total idiot for dropping out but maybe I can try it again sometime. Fingers crossed for Orserdu!
2 -
I've been out-of-the loop on BCO for a long time, but thought I'd share this.
The understanding of cancer seems to be changing leading to new ideas about treatments. About 3 days ago Tucker Carlson interviewed Dr. Patrick Soon-Shiong about his work on cancer vaccines, one of which is based on the BCG vaccine. A long interview, but I found it facinating and hopeful. I went to Dr. Soon-Shiong's ImmunityBio company website to check out their current clinical trials. Unfortunately there are none for Breast Cancer, but you can still fill out an inquiry form for "Clinical Trials for Other Cancers" which I did. I know of another oncologist/researcher (Dr. Angus Dalgleish) who has also developed a cancer vaccine based on BCG. Dr. Dalgleish works with melanoma patients and has seen long term remission with the vaccine, but he can't find the necessary funding for mass production.
1 -
welcome back, WeninWI- how was the trip to Arizona?!
0 -
@cblaurenceauthor good luck and fingers crossed for the Oserdu treatments ! ❤️ I so appreciated your sharing and courage.
@weninwi at the TN MBC zoom call on Friday, Melissa / mods shared this article on vaccine findings and it’s also hopeful news:
1 -
Thanks @rlschaller — glad it was helpful. Best of luck with your new treatment as well. :)
1 -
Anyone familiar with the REPLOT trial (NCT06408168)? It is specifically for ++- metastatic lobular breast cancer patients who have have had at least one prior line of a CDK4/6 inhibitor. It's being run by Dr. Mouabbi only at MD Anderson in Houston. It's testing a ROS1 inhibitor, repotrectinib, as either a single agent or in combination with fulvestrant. Repotrectinib is already FDA approved to treat some metastatic non-small cell lung cancer, so it's safety profile and side effects are already well-known. Looks like a small study, but at least the write up makes it sounds like it has some good potential.
0 -
@bighubs Dr. M is a very renowned lobular specialist, and although I’ve heard of the trial, I haven’t really read up on it. If you go to the Lobular Breast Cancer Alliance website and look at their clinical trials, it would probably give you the same information, but it’s a great resource.
I wouldn’t hesitate to contact Dr. M. Many go to him for second opinions.
Also, if you look at LBCA website, he has video presentations and is a scientific board member for the group.
I hope your wife is still doing okay on first line.
Edit: Oops. I see you’ve already been to the LBCA site. Sorry about that.0 -
@kbl,
Yes, that is where I first saw the link to the lobular specific trial. I don't know if the results of the trials for lung cancer when they were getting it approved initially bear any relation to how well lobular breast cancer might respond, but for the lung cancer they were treating with it looks like it got around a 34 month progression free survival period. That seems really long. The study size for his trial is small and only located there, and I think they want you to have had at least one line of CDK 4/6 inhibitor and progressed on that before you are eligible to join, so my wife wouldn't be there yet anyway as she's still on first line, but just thinking of what might be next.
As a general question, for those who have participated in trials, who covers all the costs? I'm assuming the trial organizers cover the healthcare costs since I assume insurance will balk at paying for an unproven therapy, but is the individual on the hook for travel and transportation costs or do the pharmaceutical companies chip in for that at all since you're doing them a favor by enrolling?
We got a second opinion at MD Anderson when she was first diagnosed, but it wasn't with this particular oncologist.
0 -
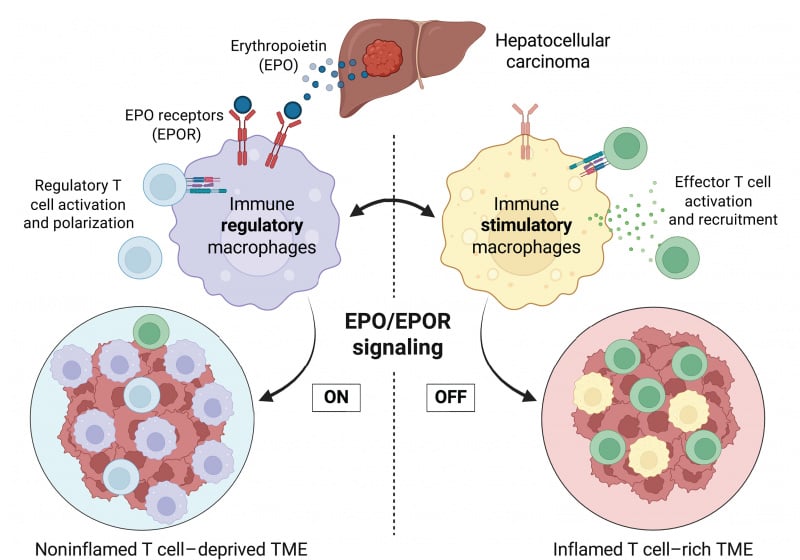
An interesting paper was just published in Science that may lead to a breakthrough in efforts to convert cold tumors (aka Metastatic Breast Cancers) to hot tumors that can be treated with immunotherapy:
A group at Stanford made the surprising finding that cold tumors, but not hot ones, secrete erythropoietin (EPO), a hormone most studied for its role in red blood cell formation. High levels of EPO attract immune-suppressing cells like macrophages and regulatory T cells (Tregs) that shield the tumor from the immune system.
0 -
Blocking the activity of the protein turns formerly "cold," or immune-resistant, liver tumors in mice into "hot" tumors teeming with cancer-fighting immune cells. When combined with an immunotherapy that further activates these immune cells against the cancer, the treatment led to complete regression of existing liver tumors in most mice. Treated animals lived for the duration of the experiment. In contrast, control animals survived only a few weeks.
This is a fundamental breakthrough in our understanding of how the immune system is turned off and on in cancer," said Edgar Engleman, MD, PhD, a professor of pathology and of medicine. "I could not be more excited about this discovery, and I hope treatments that target the mechanism we uncovered will quickly move forward to human trials."
"It's simple," Engleman said. "If you remove this EPO signaling, either by lowering the hormone levels or by blocking the receptors on the macrophages, you don't just get a reduction in tumor growth, you get tumor regression along with sensitivity to anti-PD-1treatment."
"I continue to be amazed by this finding," Engleman said. "Not every tumor is going to respond in the same way, but I'm very optimistic that this discovery will lead to powerful new cancer therapies."
Here is a summary of the work from ScienceDaily:https://www.sciencedaily.com/releases/2025/04/250424165403.htm
And the manuscript: https://www.science.org/doi/10.1126/science.adr3026
2 -
Another interesting finding comes from studies of PCSK9, an enzyme that targets specific liver cell surface receptors for destruction. Monoclonal antibodies against PCSK9 are used for cholesterol control (the drugs are called Evolocumab and Pralucent) in patients who can't tolerate statins or those for whom statins are not sufficient. These monoclonal antibodies inhibit PCSK9 activity, which results in a large increase in the numbers of LDL-c Receptor (the receptor that binds bad cholesterol). In short, injection of the antibody inhibits PCSK9 and increases the numbers of LDL receptors on the surface of the liver, which can then grab more LDL out of the bloodstream and send it for destruction in the liver, resulting in a big drop in LDL levels in the blood
Importantly, a 2005 study in Nature showed that the PCSK9 antibodies also have other roles in the body, including some that may make the tumors much more sensitive to immunotherapy. First, they found that PCSK9 also controls levels of the MHC Class I receptor on the surface of the tumor. MHC-Class I receptors are responsible for bringing neoantigens (screwy proteins that only cancer cells make) to the surface of the tumor, which attracts T cells are to come in and destroy the tumor. Tumors have ways to shut down the numbers of MHC-Class I receptors in an attempt to hide from the immune system, so the PCSK9 antibodies can counteract that. In addition, the PCSK9 antibodies were found to modulate the tumor microenvironment to convert pro-tumor macrophages (which shield the cancer ) to anti-tumor macrophages (which do not protect the cancer) and also can help with T cell activation. Consequently, clinical trials have begun to see if these cholesterol-fighting medicines may help make solid tumors respond better to immunotherapy.
0 -
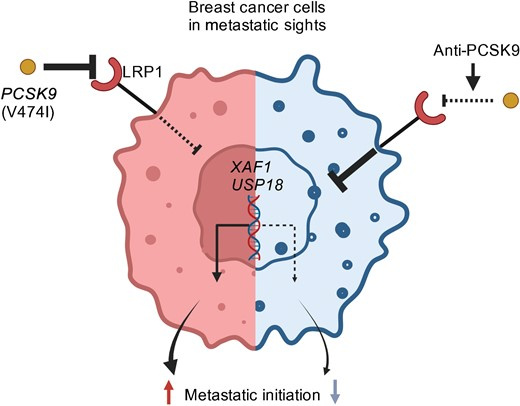
In addition, a paper that came out this past January in Cell identified an important role for PCSK9 in promoting breast cancer metastasis. This story is a bit more complicated so I will try to put it clearly…
This group was interested to find that a single amino acid variant of PCSK9, which is found in 70% of europeans (in the press release they say 70% of white females, but is also common in many other populations), is associated with a 22% chance that a breast cancer will become metastatic within a 15 year window of time, compared to only a 2% chance of developing mets for those with cancers that do not metastasize.
In trying to understand why this happens, they found that the PCSK9 variant targets the LRP1 receptor for destruction, and that doing so leads to a big increase in metastasis-colonizing genes, specifically those needed to initiate new metastases.
They went on to show that treating cells and tumor model systems with the PCSK9 antibody Evolocumab, which inhibits PCSK9, lead to increased LRP1 receptor on the cells and shut off the metastasis-inducing genes, leading to a major drop in the ability of cancers to become metastatic.
They further found in their model that Evolocumab could also reduce pre-existing metastases, to a "modest but significant" extent, but that preventing new mets from forming was the main (ie, larger) effect they saw in mice treated with the drug. Based on these findings, they recommend that trials be carried out on those with high risk early stage tumors to see if those with the PCSK9 variant would be protected from developing mets by taking Evolocumab.
The diagram shows how Anti-PCSK9 antibodies can prevent formation of new mets:
1 -
So to summarize that part, the FDA-approved drugs Evolocumab and Praluent, used to treat people with high cholesterol, may also find a role in preventing mets in those with early stage cancers and in sensitizing MBC to immunotherapy, very cool…
0 -
@cure-ious My head is spinning a bit, but great info!
As a side note, my TMB went from 5 to now 15 since the Bria-IMT trial. We also found BRCA1 promoter methylation which opens up a PARP inhibitor (I think that's what he said). When I was on the trial, I was annoyed because clearly it wasn't going to work for me (neg PD-L1) but I think it was you, cure-ious, who said it might make me sensitive to immunotherapy. I don't know if that's what happened, but it could be!
I had a grade 3 full body rash reaction to the full dose Orserdu. After three weeks, I'm on a reduced dose (2/3rds) and we'll see if that works. He said we'll 'manage' the rash if it comes back, but I don't know if I'll bother. Seems like slathering ointment all over my body twice a day and staining sheets, clothes, furniture, etc., won't be worth it. We shall see.
Hope everyone is well here!
CBL
0 -
CBL, reduced BRCA due to promoter methylation created a whole new line of therapy in PARPi for you, how wonderful!!
It might also be responsible for the TMB increase, but for full BRCA1 loss they have seen it can lead to a reduced response to immunotherapy- however they are finding combination therapies that may make it work for those with BRCA mutations, so keep a close eye on that literature. My MOs keep saying the side effects they sometimes see from immunotherapy can be permanent, so they recommend waiting a bit if possible as the data shakes out on all of it.
Orserdu is a really great med, so hopefully the lower dose fixes the problem!
0 -
Ooh, I'll have to look for that. Thank you!
Ugh. Permanent side effects do not sound like fun.
Hope you are well!
0 -
@cure-ious wow such wonderful information! Thank you for sharing. I hope the advances can be sustained given this research adverse administration. Love that new combinations might increase sensitizing for MBC to immunotherapy, very cool indeed. I could use that myself, as both monoclonal antibodies and immunotherapy did not work for me. Looking forward to new drug combos! Meanwhile my MO is staying with the old guard drugs for now, will start Doxil in 2 weeks. How are you doing on the trial you started ?
Cbl - I’ve had full body rashes in the past, and they are not fun but managed well, perhaps worth it and not all are permanent, you can see how it goes of course. The body is such a fascinating mechanism, we are constantly asked to adapt as our immune systems adapt. I also hope the lower does solves this reaction!0 -
rls & cbl, I'm doing fine thanks, my liver enzymes popped up out of normal range after two months in the trial, it was just grade 1 so the docs felt we should just watch & wait till next visit, however the pharma guys were not so chill about the first patient taking their (hoped-for) potential multi-billion $$$ drug having liver problems just two months into the trial, as you can imagine. They asked me to drop the old cholesterol drug they had switched me to at the start of the trial (because their drug is not compatible with statins), and go in for more labwork to recheck liver numbers asap. Fortunately that did the trick, and the enzymes took a dive right back to normal, so I imagine they were doing high-fives all around. My doc here then put me on Praluent (which is one of the antibodies for cholesterol mentioned above) and of course I immediately went on a deep dive to see if this drug might have any anti-cancer activities in addition to lowering LDL, and was happy to see that it might help in a couple different ways. Its probably not particularly relevant for me in this trial, but will be tracking it to see if it helps immunotherapy in the ongoing trials.
2 -
Dear Cureious, thank you - very interesting about PCSK9. So, looks like one drug shoots two targets at a time (cholesterol&metastasis control). Do you think Evolocumab/Praluent could exchange Tamoxifen for EBC HR+ population, and even become preventive drugs for EBC HER2+ and TN (wouldn't that be great as these two populations did not have prevention after treatment)? Saulius
0 -
Hi Saulius,
Its interesting that PCSK9 inhibitors seem to work similarly in triple-negative, Her2-positive, and Er-positive subtypes, and the effect on preventing new mets or colonization of mets in distant organs is significant in pre-clinical studies, much more so than the small effects they see on existing mets.
More relevant for us is the big effects they see, again in pre-clinical trials, on helping immunotherapy work, and that of course is applicable for MBC patients. I also saw a review on the heart issues that concluded based on multiple metabolic changes that these drugs may be cardioprotective for patients taking checkpoint inhibitors. Heart side effects from immunotherapy are considered rare (1%) but some reports say half of those can be fatal even in the first couple weeks, I guess the activated immune cells infiltrate the heart and its like you are experiencing a heart transplant rejection. Given the severity of the potential problem, I'd say a 1% chance doesn't sound so very rare, so its good to know this could help people with heart issues safely take Keytruda.
I'm curious about this variant of PCSK9 that is seen in certain populations (70% of europeans, a large number in east Asia and another in North Africa, etc) and predisposes people who get cancers to develop mets. It certainly didn't get that widespread in the population because it wanted to increase the number of people with cancer to develop mets! There must be other gain of function effects from that mutation, and I wonder if those with MBC are even more likely to have that mutation, and consequently those with MBC might benefit even more than the population at large from trying PCSK9 inhibitors in combination with immunotherapy, regardless of which subtype. For me the conclusion is I would want to be taking it if I tried immunotherapy. In colon cancers its reported that PCSK9 stimulates Akt in the PIK3CA/AKT pathway, so inhibition might also help those with PIK3CA mutations, for example. But for now I just see four clinical trials for PCSK9 inhibitors in cancer, and all are lung cancer with immunotherapy… So it will take awhile to know what any benefits might be for MBC specifically.
1 -
Hi everyone,
I came by to check in to see if you'd posted an update on how your trial is going cure-ious - I'm glad to read that you are still in it even after the the wobble in liver #s. Keep on keeping on…
I was disappointed that I am ineligible for the same trial in Toronto because of two of my previous lines of treatment (inavolisib and everolimus). Right now I'm on a break from Enhertu. Can't say I'm noticeably less tired than usual, though it does seem that my fitbit steps are creeping up. But that might be the improving weather. I certainly haven't missed the fuss of blood tests and infusions or the tired/wired feeling for the couple days following.
I find out next week what my oncologist thinks my next step should be - likely restarting:(
Good luck to all.
0 -
“Thank you for your email and apologies for the delayed response.
The Rolo trial was presented at a conference in December and unfortunately crizotinib had no activity at all. Apologies if the websites you have viewed were not up to date with this information, this should be rectified soon.
Best wishes
Orla Batchelor
Senior Trial Manager – Breast Portfolio
The Royal Marsden Clinical Trials Unit (RM-CTU)
The Royal Marsden NHS Foundation Trust”
Not holding my breath for signal in the Repotrectinib trial at MDA either.0 -
Hi NewGardner,
The BriaCell IMT vaccine plus immunotherapy trial is now in phase 3 and has sites in Canada, is that an option?
1 -
Dear cure-ious - I haven't followed that trial, but if it is this side of the border maybe I should contemplate it. Thank you for the suggestion.
0 -
New Gardener, You just reminded me, I think the company also will be providing this treatment to MOs once their phase 3 trial fills up, that they would be able to request it for compassionate use if a trial is not available. Lemme go look for that and their latest data.
0