Are you currently (or have you been) in a Clinical Trial?
Comments
-
Veru has opened another clinical trial for the AR agonist, Enobosarm, in combination with Verzenio (CDK4,6 inhibitor). Phase 3, designed for those who progressed on Ibrance-Femara/Faslodex. Comparative is Exemestane-Everolimus.
https://clinicaltrials.gov/ct2/show/NCT05065411
0 -
sounds like a promising study- I hope it will be another option down the line
0 -
Has anyone heard about this trial? My onc wants me to consider it. It has many locations and they're all actively recruiting. It requires a treatment change before progression which is novel and nerve-racking.
Phase III Study to Assess AZD9833+ CDK4/6 Inhibitor in HR+/HER2-MBC With Detectable ESR1m Before Progression (SERENA-6) (SERENA-6)
0 -
This is the trial that was recommended for me today by my MO. It uses an Antibody Drug Conjugate and people with a few various cancers are eligible, including TNMBC, which is me. It’s only at Phase 1, unfortunately, so no data available yet. My current chemo has had only mixed results on my liver mets and hasn’t helped my spine mets at all, at least one of which is still ER+. Any thoughts? I’m leaning toward saying yes.
0 -
rosie24,
That trial looks very interesting and looks like it's pretty much brand new. Glad to see so many new advanced solid tumor trials open to TNBC.
Hugs, Susan
0 -
Thx for responding, Susan. Yes, I was happy to see some focus ontreating triple negative BC also.
0 -
Hi Rosie24 - I'm in a clinical trial for a different ADC (for enfortumab vedotin, it's approved for urothelial cancers, NCT04225117). I really liked the concept of the targeted delivery, but I've since discovered that there are some system-wide side effects nonetheless. But it's my 12th line of treatment and the first scan showed stability, which is good news of course:)
I found this article on ADCs interesting - though most was well above my paygrade. I checked and the compound in your trial isn't mentioned, but the overarching discussion still applies.
https://acsjournals.onlinelibrary.wiley.com/doi/ep...
Good luck!
0 -
Newgardener, Thanks for the link. Same for me, with being above my pay grade, but I was able to learn a bit more on how ADCs work. I’m hopeful for this new drug to do good things, and hope yours does too. I expect to start on 6/20 if all the pretesting goes well.
0 -
An exciting report in Lancet describing the results of phase 2 trials for the AKT inhibitor Capivasertib, tested with Faslodex in the FAKTION trial. Participants were allowed up to 3 prior endocrine therapies and one chemo in the metastatic setting. The results show clear PFS as well as OS benefit for Capivasertib, but only for the cancers that have PI3KCA mutations (also benefit for cancers with AKT activating mutations or PTEN loss, these all affect the same pathway). These patients had not had CDK4,6 inhibitors, because the trial started in 2015. No prior use of Faslodex, PI3KCA drugs like Piqray, or mTOR inhibitors (like Everolimus) was allowed in this trial.
Looking at the graph below, in (B) you see that the PFS for people who took Faslodex with Capivasertib (red line) was much better than the control group with Faslodex alone (blue line). The group here all had cancers with either PI3K3CA mutation, or AKT mutation or PTEN loss. (Note the bottom got cut off somehow, but it is the same time scale in months as in graph D) In Graph (C) you see that PFS was not demonstrably better for those who got the drug (red) vs the control group (blue) in the subgroup of people who did not have a PI3KCA mutation. In graph (D) you again see a significant overall survival (OS) for the group who took the drug (red line) versus control (blue line), and again this was the group that had PI3KCA pathway alterations. The graph (E) shows there was no OS benefit for the people in the study with cancers that did not have PI3KCA mutations.
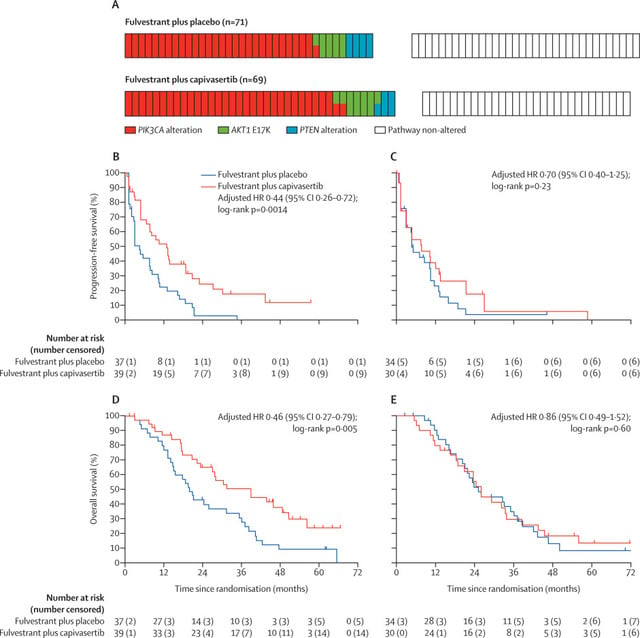
The authors point out that this is the first drug to deliver an overall survival advantage for PI3KCA mutant MBC, this was not seen in trials for Piqray (Alpelisib) or Everolimus. So this is a promising new drug for cancers with PI3KCA mutations!
Here is a link to the paper: https://www.thelancet.com/journals/lanonc/article/...(22)00284-4/fulltext
0 -
Here is a Phase 3 trial, with Capivasertib and Fulvestrant. No prior SERDs, AKT, PI3K, mTOR, or Fulvestrant allowed.
https://clinicaltrials.gov/ct2/show/NCT04305496
And another Phase 3 trial, combining Capivasertib (AKT inhibitor), Fulvestrant (Faslodex) and Ibrance. No prior SERDs, AKT, PI3K, mTOR, or CDK4,6 inhibitors allowed in the metastatic setting.
https://clinicaltrials.gov/ct2/show/NCT04862663
and a summary from last year: https://www.icr.ac.uk/news-archive/breast-cancer-d...
The trials do not select for those with PI3KCA mutations, but the new Lancet paper just published shows only those cancers get the benefit..
0 -
Cure-ious,
Looking at the Phase III trial with Fulvestrant, there are 276 sites but only the China sites are recruiting. The majority of the other sites say "Withdrawn". Any ideas why this would be?
Hugs, Susan
0 -
Hi Susan,
Dr Rugo mentioned this clinical trial is starting at UCSF this month -->Capivasertib (AKT inhibitor), Fulvestrant (Faslodex) and Ibrance
0 -
Werone,
Thanks for letting me know! Looks like I would qualify for the Phase Ib part of the trial but since I have already been on Piqray, Ibrance and Faslodex I kind of doubt it would work. I'll ask Hope about it the next time I see her.
Hugs, Susan
0 -
I follow this Thread for research. I am cross posting from my post from another Thread. What do you all think of this...... Cure-ious thank you for posting links for me to read and learn about. Thanks to all on here that want to know the latest cancer news. Here is what I just posted on another Thread-----
Ok I had a surprising, frustrating, and upsetting Palliative Care video appointment today.
I told the Palliative Care doc about my disappointing first office visit with a new rheumatologist (long story) and how I posed the question to the rheumatologist about the possibility for me to use immunotherapy in my future for the cancer with my documented autoimmune disorders. The rheumatologist did not answer me. I explained to Palliative Care that I read and research cancer treatments and read that Trodelvy (an immunotherapy) was just approved for HR+/HER2- MBC-- previously just for Triple Negative. My Palliative Care doc said "the Lynparza is working". I said "Yes, but I am thinking about future treatments".
Then the Palliative Care doc surprised me by saying my MO will decide, at that time of progression, what my next treatment will be. And if my MO has questions on if the treatment is right for me, she will consult her staff of colleagues and make the decision with them. And the Social Worker that is in my Palliative Care meetings also agreed.
WHAT ??!!
We talk on here (BCO) about new research, clinical trials, and we post links to articles. We have a Thread dedicated to "Clinical Trials". I just read on one Thread on here today about the new cancer drug for rectal cancer being a game changer. Good grief, even those damn commercials we loathe talk about "ask your doctor if _____ could be right for you". But, now my Palliative Care doc says that I am stressing too much, researching too much, and to have faith in my doctors to make the right decisions for my cancer. The Social Worker said I am being too anxious also.
I am tired and frustrated. I think I will just keep my mouth shut from now on.
0 -
Dear Candy, these doctors usually don't understand how restless, anxious and depressed we are. I don't remember who compared being stage IV with standing with your feet in concrete and a bus without brakes rushing down the hill to you... I have heard too many times this simplistic "why are you asking this, we are doctors - we decide, you go smile and live your life". I think doctors are so overloaded these days that they mostly care about procedures and have no time for feelings... sad though, especially when this happens in palliative care:/
Saulius
0 -
Candy - my approach is to 'trust, but (self) verify' with my onc. When progression came I had a pretty good idea about the options open to me and which I would likely prefer (thanks also to you going before me!). So when onc came up with three options for us to discuss I more or less knew I wanted to try Lynparza and it was her preference as well. In the meantime, Ill read and research and keep things in the back of my mind so when we hit the next progression point Ill be educated enough to question and challenge and make an educated decision with my team.
I hate that the language that they used makes it sound like they are dismissing your concerns and knowledge, but perhaps what is really meant is more along the lines of 'well, why worry now/make a decision when who knows what the cancer is going to look like and where at progression'. We all know how that story goes with receptors flipping etc. So while its good to have existing knowledge and build on it for all eventualities to discuss at the right time, I think MOs just don't have time in their day to discuss something in the future, though they certainly will at the time.
That being said, though, I would have thought your rheum would have had a position one way or another on immunotherapy drugs, even at a high level. I know thats been your concern in the past - if certain treatments are even an option for you given your other conditions. Just getting that concern relieved would have been helpful!
0 -
Candy,
I applaud your efforts to take control of your care. Your doctors should appreciate the feedback and knowledge you share. No one cares more about your treatment than you so you have to be at the table.
I also wanted to mention that Trodelvy is not immunotherapy. It is an antibody drug conjugate (ADC). Specifically, it consists of a Trop II directed antibody, delivering a Top I inhibitor SN-38. I was able to stay on it for a year with no bad SEs despite being heavily pretreated. ADCs will revolutionize cancer care as the CDK inhibitors like Ibrance and Verzenio did six years ago.
Hugs, Susan
0 -
Susan- Interesting. I have so much to learn. I thought Trodelvy, and antibody drug conjugates, were immunotherapy. Working with the immune system to fight the cancer. See, that is why I need my docs-- MO and rheumy-- to say "No, Candy, that is not immunotherapy, and you are still a candidate for those treatments if we need them in the future". I will read/ research more, but this is way over my head.
Anybody else, Cure-ious for instance, that can explain if antibody drug conjugates are immunotherapy. And if contraindicated in someone with autoimmune disorders??? I need advise from this group since my docs do not seem to want to have a discussion with me about this.
0 -
Candy,
ADCs are a class of drugs with an antibody, linker and toxic payload. The payload can be an immunotherapy, such as the experimental ADC, BDC-1001. Trodelvy does not deliver an immunotherapy payload.
0 -
Susaninsf- After I posted I Googled about Trudelvy. Some sites state it is an immunotherapy of sorts. That it is an "immune targeted therapy".
"Trodelvy's antibody attaches to a specific protein on the surface of cancer cells. This flags the cancer cells so that the immune system can detect and help destroy them."- a quote from 1 site. So the body's immune system can get involved.
"...doctors have not known whether immunotherapy is safe and effective for people who have both cancer and an autoimmune disease, because such patients have been excluded from clinical trials of immunotherapy drugs." was what I found when posing the question if someone with autoimmune disorders can use immunotherapy.
"Immunotherapy may intensify autoimmune disease symptoms"---another quote I found.
"Patients with pre-existing AD (AD= autoimmune disease) (e.g., lupus, rheumatoid arthritis, IBD, and many other disorders) are considered to have a very high risk of experiencing flares of their AD when treated with ICIs (ICIs= immune checkpoint inhibitors) or perhaps to develop other organ-specific inflammation because of their predilection toward autoimmunity."--another quote I found.
So it is sounding like immunotherapy, immune checkpoint inhibitors, or immune targeted therapy would not be safe for me. But that is my interpretation of what I am Googling. I cannot get an answer from my rheumatologist or my MO as of yet.
0 -
I am on Trodelvy and in a group on FB and everyone including my MO calls it a chemo and that is what I am seeing also on line. It targets Trop2..but it works like a chemo....and lets remember complete hair loss as well...
0 -
That is why I am looking for a discussion with my docs, to clarify this. Am I a candidate for these treatments for the future? Trodelvy, Keytruda, Enhertu, Antibody Drug Conjugates, or any others of this type that comes to market,,, or clinical trials. Or is it "No, we cannot use these in you due to your autoimmune conditions. It would be too unsafe". But I cannot start a conversation with them. Not yet anyway.
0 -
Herceptin does something similar to Trodelvy, ie "flags" the receptors,making them more visible so that your own immune system can "see" them and fight them. But that is not the same action as immunotherapy, which typically stimulates your immune system and causes the cytokine storm and the other potentially dangerous effects. Herceptin and Trodelvy are not causing any additional immune action, they are simply preventing the cancer cells from hiding and making them vulnerable to your body's immune action. Additionally, Trodelvy is delivering chemo directly to the cells it targets. Hope that makes sense.
Not saying it is or isn't safe in your particular case Candy, since I am not a physician, but just describing the action of the drug.
0 -
Yea ...Candy I totally do NOT see Trodelvy as an immunotherapy at all...in all the research and info about the drug I see..its a chemo. I do not qualify for immunotherapy as I am PDL1 negative so this also wouldn't be covered for me as a treatment if it was an immunotherapy
0 -
It is good to read what you all are posting. I do hope that these drugs can be used in my future. I have used Ibrance/Letrozole, and now Lynparza. I am just thinking of my future. Making a list, so to speak. I do not have PIC3CA so that targeted therapy will not work. The only actionable mutation I had was BRCA. I flipped from ER+ to ER- with my last biopsy. Just wondering about the "newer" treatments I am hearing about--- Trodelvy and Enhertu as examples. Lots in the news right now about them. Just wonder if I qualify for them with my autoimmune comorbidities. And I wish my docs were more receptive to discussing my options. They seem to want to wait, for now, as the Lynparza is still working. But, we know, it won't work forever.
0 -
Well here is a question Candy - I know sometimes people reach out to the drug folks or the study people for more information on a drug/study - is that something you could do to be proactive around your medical team? Someone correct me if Im wrong about there being a point of contact for clinical stuff, I feel like this has been done before by folks on this board.
0 -
Candy,
For sure, you should consult with your doctors. There is still so much they don't know about AI diseases. Have you seen this NIH article? Cancer and Autoimmune Diseases.
Although I don't have an AI disease, I had severe grade 3+ mucositis after being on Keytruda for just a couple of months. At one point, I couldn't even drink water without pain. It took a year and a half for that to go away. Not going to go on any ICIs again.
Hugs, Susan
0 -
Susaninsf- Yes, I did see that article when searching the topic. I saved it. The link noted in the article, I cannot tell you which right now or I will lose my post, went to a clinical trial on the subject. I don't know if it would be for MBC or not. The trial is being conducted at my cancer center, among others.
Sondra- I know there is a contact email/phone number usually on the clinical trial info, I have not contacted anyone yet, though. I wanted to start with my established docs first and have the conversation with them.
0 -
I have written to and called a number trial contacts and only one has gotten back to me, for the NIH/NCI T-cell trial. A BC friend asked Stanford about why no one was getting back to me about one of their trials and was told that I need to go through a referral from my MO.
0 -
My friend with TNBC is looking into a trial at MD Anderson for Larotrectinib. When I Googled the drug, it was given approval back in 2018. It targets NTRK mutations, which she has. The article says that it was the second drug to be approved for a specific genetic change, not the location of the primary cancer. Keytruda was the first.
Has anyone been on this trial or the drug?
Thanks, Susan
0