Breaking Research News from sources other than Breastcancer.org
Comments
-
I registered on the lab Corp website. Their doctor writes the order and you go to a Lab Corp location for the blood draw.
Covered by myinsurance except for the small website fee $15 I think it was.
Link - https://www.labcorp.com/coronavirus-disease-covid-19/individuals/antibody-test
Lumpie wow >2500 that's great. My sister did hers at the same time and her result was over 700. My infusion nurse said hers was only in the 200s0 -
Ditto. My doc would not prescribe, that is why I did the "no order in hand" option through LabCorp. As Olma61 says, register, if you have not already done so, on LabCorp's site. Then you have to find the correct test. It can be a little tricky. You can find it in the test menu (one of the tabs near the top of the main page). Their doc will write the Rx for the test if your doc has not provided one. They charged me a $6 fee for that service. It is not an at home test. You must go to LabCorp for a blood draw. Results are delivered through the LabCorp website. Interestingly, my insurance did pay for the test. I wa surprised.
Test name is "SARS-CoV-2 Semi-Quant Total Ab" (if seacrching by name, I had to shorten the name to "SARS-CoV-2 Semi-Quant" to get anything to come up.)
Test # is 164090
There is another very similarly named test for IgG rather than "total" antibodies. The descriptions do not make it entirely clear why you'd want total rather than IgG. I had the impression that back before the vaccines, the IgG test was an indicator of prior but cleared infection. I suppose the "total" test might count IgM, too. But I am not entirely sure about this reasoning/analysis. I ordered the above because it made sense to me and was the one that was recommended through my local MBC cancer buddies network.
Note: I have been advised that you should wait at least 30 after getting a vaccine or booster to have this test. I waited >90 days.
I was very pleased with the >2500, especially since I am on active chemo. An MBC buddy said hers were around 900. She is not on active chemo but gets a targeted therapy and is otherwise in good health. I think that some of the hard-to-account for discrepancies in results are one reason clinicians have some concerns about relying too heavily on this test. I think it is fair to say it just gives us a sense of where we are on a given day.... and it is a snapshot since the studies seem to be indicating that our immunity may wane over time... hense all the boosters....
Good luck and stay healthy out there!
0 -
Thanks for the information Lumpie and Olma61
0 -
How do you know if you are Luminal A or Luminal B or other ?
thanks
0 -
this article helps explain those subtypes
https://www.breastcancer.org/symptoms/types/molecular-subtypes
0 -
olma61-thanks. Very clear article, a k67 was never done on my original cancer biopsy, so I guess I am never going to know whether mine is A or B.
0 -
Nkb me either, but it did say that A is typically lower grade, which mine was, so I'm assuming I'm A. Not much difference between A & B anyway
0 -
Nkb, I always understood that if you are hormone positive and HER2 negative, you are Luminal A; hormone positive and HER2 positive, you are Luminal B. You probably know that from your patho report. I suppose that if you are HER2 negative with high levels of Ki-67, but they did not comment on Ki-67 in your patho report, that could present a twist (uncertainty), but in most cases, I think your patho report will give you this info. Things change over time, especially with the emergence of genomic analysis, so maybe this is over- simplified. Hope you find some certainty/reassurance.
0 -
Lumpie- thanks. At the appt to discuss the path report (10 years ago) I asked about k67 and the surgeon said it wasn’t done. That they weren’t doing them as much. I heard that many MOs don’t find them as consistent so don’t do them. I was Her2 negative but recently looked again and was 2+ so basically Her 2 low.
I’m not stressed, just curious. The only mutation I have that Iknow of (CCND1) wasn’t listed in the article- but, overall super interesting article.
0 -
Nkb, did they ever test your HER2 by FISH? Mine was also 2+ by IHC so they sent it for further analysis by a more sophisticated test “FISH”. I was still in the grey zone and had several pathologists weigh in on whether I’d benefit from HER2 targeted therapy, ultimately it was decided that I should be treated as negative. If they never tested your HER2 status beyond the equivocal 2+, that might be something to ask your MO about. The HER2 targeted therapies have experienced great advancements and so it seems more important than ever to take a closer look at cases that might be positive by FISH
0 -
There is some overlap between the luminals & hormone markers but it's not a 100%. for ex this study below found that some triple negs were not basal.
& in metastatic cancer, there is a difference in survival curves for luminal a, luminal b and basal
"Median OS was 99.7 months for luminal A, 63.6 for luminal B, 34.7 months for HER2-enriched, and 22.4 months for basal-like."
https://community.breastcancer.org/forum/73/topics...
0 -
Triple Neg has other subtypes too... 4-6 depending who is grouping them. If you go a genetic assay scattergram you can see the clusters of the different types but multiple genes control it.
basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M), mesenchymal stem–like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR)
0 -
JennyJo20- yes, they FISH tested it and it was negative.
I’ve heard that Herceptin is very weak compared to for example Enhertu- and doesn’t work on Her 2 low- but Enhertu works on about 35% of Her2 low.
My UCSF second option MO says in biopsying again for an Enhertu study no one who was 2+ at primary turned negative although some 1+ people did.
Interesting about all the triple negative subtypes.
0 -
Probiotic Supplement Attenuates Chemotherapy-Related Cognitive Impairment in Patients With Breast Cancer
- This randomized, double-blind, placebo-controlled trial showed that a probiotic supplement attenuated the incidence of chemotherapy-related cognitive impairment (CRCI) in patients with stage I–III breast cancer who underwent chemotherapy. No measurable side effects were found during the treatment. Disease-free survival and death rates in the probiotic group were slightly better numerically but not significantly than those in the placebo group.
- Probiotic supplementation during chemotherapy is a simple, inexpensive, safe, and effective intervention for the prevention of CRCI. Impact on long-term outcomes or survival warrants further trial study with a large sample size.
Article: European Journal of CancerProbiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: a randomised, double-blind, and placebo-controlled trial
Eur. J. Cancer 2022 Jan 01;161(xx)10-22, Z Juan, J Chen, B Ding, L Yongping, K Liu, L Wang, Y Le, Q Liao, J Shi, J Huang, Y Wu, D Ma, W Ouyang, J TongDOI:https://doi.org/10.1016/j.ejca.2021.11.006Abstract: https://www.ejcancer.com/article/S0959-8049(21)01216-8/fulltext{Reporting and abstract: free access; full journal article requires fee or subscription. IDK about you, but any news about improving on chemo brian is welcome to me! I have been taking a probiotic to help with intestinal distress for almost a year now. It has helped my intestinal situation dramatically. My chemo brain has also improved. I thouht it was time/coincidence, but maybe the probiotic is improving things.}0 -
A new paper has been published about ErSO. From the abstract...
"While ErSO has promise as a new drug, it has effects on ERα-negative (ERα−) cells in certain contexts. Herein, we construct modified versions of ErSO and identify variants with enhanced differential activity between ERα+ and ERα– cells. We report ErSO-DFP, a compound that maintains antitumor efficacy, has enhanced selectivity for ERα+ cancer cells, and is well tolerated in rodents."
Unfortunately, still no signs it will make it into clinical trials in the near term.
https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c01...
0 -
New mammogram measures of breast cancer risk could revolutionise screening
Abstract/What's new? dtd 16 November 2020
Mammographic density, or the area of the mammogram which appears white or bright, has well-established associations with breast cancer risk. The authors call this Cumulus due to the computer-assisted technique for measuring that density. Here, the authors introduce two novel measurement techniques, Cirrus and Cirrocumulus, for extracting risk information from mammograms. Cirrocumulus is based on image brightness and Cirrus is based on texture. When combined, these measures substantially improve risk prediction beyond that of Cumulus. In addition, the new risk measures outperformed the recently published polygenic risk score. By obtaining more information from mammograms, these tools could improve personalized risk recommendations for screening.
Reporting: dtd 23 Dec 2020
World-first techniques for predicting breast cancer risk from mammograms that were developed in Melbourne could revolutionise breast screening by allowing it to be tailored to women at minimal extra cost.
Published in the International Journal of Cancer, the University of Melbourne-led study found two new mammogram-based measures of risk. When these measures are combined, they are more effective in stratifying women in terms of their risk of breast cancer than breast density and all the known genetic risk factors.
Researchers say if successfully adopted, their new measures could substantially improve screening, make it more effective in reducing mortality and less stressful for women, and therefore encourage more to be screened. They could also help address the problem of dense breasts.
Since the late 1970s, scientists have known that women with denser breasts, which shows up on a mammogram as having more white or bright regions, are more likely to be diagnosed with breast cancer and to have it missed at screening.
Collaborating with Cancer Council Victoria and BreastScreen Victoria, University of Melbourne researchers were the first to study other ways of investigating breast cancer risk using mammograms.
Using computer programs to analyse mammogram images of large numbers of women with and without breast cancer, they found two new measures for extracting risk information. Cirrocumulus is based on the image's brightest areas and Cirrus on its texture.
First, they used a semi-automated computer method to measure density at the usual, and successively higher levels of brightness to create Cirrocumulus. They then used artificial intelligence (AI) and high-speed computing to learn about new aspects of the texture (not brightness) of a mammogram that predict breast cancer risk and created Cirrus.
When their new Cirrocumulus and Cirrus measures were combined, they substantially improved risk prediction beyond that of all other known risk factors.
Lead researcher and University of Melbourne Professor John Hopper said that in terms of understanding how much women differ in their risks of breast cancer, these developments could be the most significant since the breast cancer genes BRCA1 and BRCA2 were discovered 25 years ago.
"These measures could revolutionise mammographic screening at little extra cost, as they simply use computer programs," Professor Hopper said.
"The new measures could also be combined with other risk factors collected at screening, such as family history and lifestyle factors, to provide an even stronger and holistic picture of a woman's risk.
"Tailored screening – not 'one size fits all' – could then be based on accurately identifying women at high, as well as low, risk so that their screening can be personalised.
"Given mammography is now digital, and our measures are now computerised, women could be assessed for their risk at the time of screening – automatically – and given recommendations for their future screening based on their personal risk, not just their age."
Professor Hopper said this information could be used to ease pressure on BreastScreen, which had to close for a period during the COVID-19 pandemic and is looking for ways to best handle the backlog while continuing to provide a valuable service with limited resources.
He said the current breakthrough could not have occurred without the extraordinary support his mammogram research had received from the National Breast Cancer Foundation, starting with its first funding round more than 20 years ago.
"Only around 55 per cent of Australian women aged 50-74 currently present for screening aimed at detecting breast cancers early," he said.
"Knowing that screening could also give an accurate risk prediction could encourage more women to take up the offer of free screening. Women with high risk based on their mammogram would also benefit greatly from also knowing their genetic risk."
Adjunct Associate Professor Helen Frazer, Clinical Director of St Vincent's BreastScreen Melbourne, said that improvements in assessing a woman's risk of breast cancer would be transformative for screening programs.
"Using AI developments to assess risk and personalise screening could deliver significant gains in the fight against breast cancer," Adjunct Associate Professor Frazer said.
The study involved participants in the Melbourne Collaborative Cohort Study run by Cancer Council Victoria, and the Australian Breast Cancer Family Study and Twins Research Australia run from the University of Melbourne.
Participating women filled out a questionnaire and allowed researchers to access their mammograms from BreastScreen, with other providers or their own copies.
This work was conducted by Dr Kevin Nguyen at the University of Melbourne, whose ground-breaking PhD uncovered the Cirrocumulus measure, and is a continuing collaboration with researchers from Seoul National University in South Korea.
Researchers from Monash University, University of Hawaii Cancer Center, University of Pisa and University of Western Australia also contributed to the study.
Reporting: https://about.unimelb.edu.au/newsroom/news/2020/de...
Abstract: https://onlinelibrary.wiley.com/doi/10.1002/ijc.33...
Journal: https://onlinelibrary.wiley.com/doi/full/10.1002/i...
https://doi.org/10.1002/ijc.33396
{Abstract and reporting are free. Full journal article requires fee or subscription.}
0 -
Anthracyclines for HER2+ Breast Cancer: Are We Ready to Let Them Go?
"The role of anthracyclines for early HER2-positive BC appears limited in an era of highly effective HER2-directed therapies. De-escalation of (neo)adjuvant regimens is possible and safe, by using paclitaxel and trastuzumab or trastuzumab emtansine for small tumors and by using taxane- and platinum-based neoadjuvant regimens for larger tumors, including those with high-risk features.
Omitting anthracyclines can result in a substantial reduction in potential serious toxicities, while still achieving optimal treatment outcomes. Several additional de-escalation trials are currently ongoing and may allow for even further reduction in toxicity of treatment, ultimately allowing us to maximize the benefit of chemotherapy while minimizing its undesired impact on the quality of life of our patients."
0 -
That sounds very exciting Laughing Gull, I hope that is the case!
0 -
A New Solution for Long-COVID Brain Fog?
Noninvasive brain stimulation (NIBS) that uses alternating microcurrents appears to rapidly and effectively improve cognitive and visual deficits related to "long COVID," a small case series shows.
SARS-CoV-2 infections impair blood flow to the eyes and brain, causing visual and cognitive deficits, and the stimulation restores normal blood flow....
"Oxygen and glucose is delivered again to nerve cells, so they can do their job of firing electric signals to the brain, and the patient can think better, their cognition is better, and their vision is improved."
The benefits for those affected with long-term cognitive problems occur within days.... "This Is the fastest solution that I'm aware of ― much faster than with the standard neurological rehabilitation."
The study was published in a recent issue of Restorative Neurology and Neuroscience.
Reporting: https://www.medscape.com/viewarticle/968290#vp_1
Journal article: https://content.iospress.com/articles/restorative-...
DOI: 10.3233/RNN-211249
{This is about covid brain fog, but anybody who says "brain fog" has my attention. Makes me wonder if these therapies could help those of us suffereing from chemo-related brain fog as well. We must hope (and perhaps advocate) for clinical trials. Access to reporting and journal article are both without charge. Reporting may require registration.}
0 -
That caught my eye too in terms of chemo brain, but it was a study of only two patients. Something to investigate further for sure.
0 -
"HER2-positive breast cancer progressively turned from the most feared to the most curable subtype. Currently, more than 9 out of 10 patients with HER2-positive breast cancer who are treated with chemotherapy and HER2-blockade are free from recurrence 6 years after surgery."
Woot woot!!! What great advancements!! Let's hope this becomes the norm for all subtypes asap!
More Tolerable Cures for Patients With Early-Stage HER2-Positive Breast Cancer: Halfway to Precision
https://dailynews.ascopubs.org/do/10.1200/ADN.22.2...
0 -
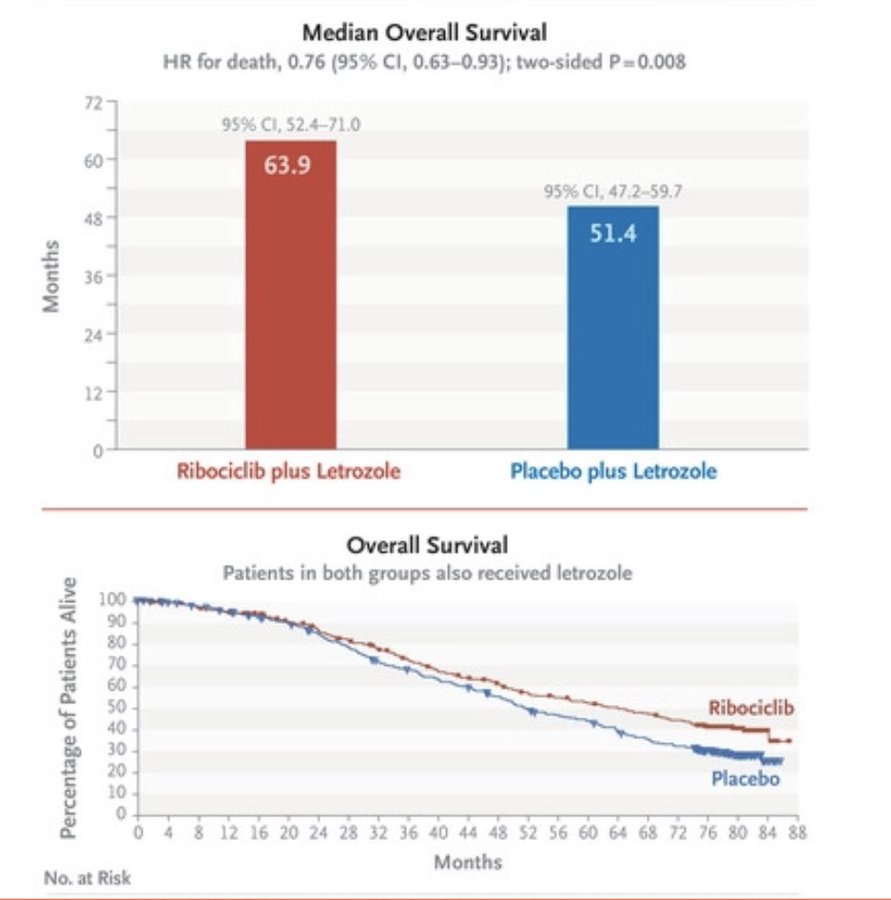
"overall survival data from MONALEESA-2, breaking the 5-year mOS barrier for patients with metastatic HR+ breast cancer"
0 -
Accelerated Partial Breast Irradiation using External-Beam or Intraoperative Electron Radiotherapy: 5 year oncological outcomes of a prospective cohort study
this is for rads post lumpectomy for IDC or DCIS
" Ipsilateral breast tumor recurrences and locoregional recurrence rates were unexpectedly high in patients treated with IOERT" 10.6% recurrence in the IORT group v 3.7% in accelerated partial breast rads
https://www.redjournal.org/article/S0360-3016(22)00243-7/fulltext
0 -
Treatment and Survivorship Interventions to Prevent Poor Body Image Outcomes in Breast Cancer Survivors
https://www.dovepress.com/treatment-and-survivorship-interventions-to-prevent-poor-body-image-ou-peer-reviewed-fulltext-article-BCTT
This might have already been posted, but I read it in detail yesterday. I think it's crucial that the BC medical community really spends time realizing that taking care of the whole person overrides what I still have seen as the knee jerk reaction to just annihilate the cancer with secondary regard to the aftermath of a woman who must live with decisions that can be made without us being prepared with the right questions or coming to an agreement with the best possible treatment.
Here are the major sections:
We Shared Decision-Making
Local Therapy De-Escalation
Axillary Downstaging
Radiation
Systemic Therapy
Lymphedema Risk Mitigation
Preventing and Managing Weight Gain During Treatment
Prehabilitation
Addressing Menopausal and Sexual Sequelae of Treatment
Psychological Interventions During Treatment
I feel strongly that these subjects need to be incorporated into the care of every newly diagnosed BC patient. I've gotten so much of this support and preparation from being here, but not everyone knows about options until they have made permanent decisions with what still seems to be a traditional approach that is a decade behind current best practices. As a person living in a city without a major breast care integrated care system, I still prefer to piece the right solution for me into what we have here. I may still end up traveling. But knowing that treating my cancer has options that require shared decisions is both empowering and unnerving for me. My BCS is in line with my approach, but for now, we may lack oncoplastic expertise that can keep be here.
0 -
[trial in mice] World first: drug prevents human breast cancer recurrence and metastasis (article headline)
From the actual paper's abstract:
"We previously provided in vitro evidence that these features are collectively enforced by mitochondrial superoxide in a paradigm where mitochondria act as metabolic sensors of the tumor microenvironment and produce subcytotoxic levels of superoxide to prime metastatic progenitor cells. We also showed that these metastatic traits can be collectively countered by MitoQ, a mitochondria-targeted antioxidant that selectively deactivates mitochondrial superoxide. Here, we further establish that MitoQ prevents primary tumor recurrence after surgery, tumor take and metastasis as a whole, notably in a model of human breast cancer in mice. Since MitoQ already successfully passed Phase I clinical trials, our findings support the development of this drug as a preventive treatment against breast cancer metastasis."
This patented substance (MitoQ), which is available OTC as a supplement, was selected for trial based on their theorized mechanism of action (suppressing subcytotoxic mitochondrial superoxide) by researchers with no connection to the company -- in part because MitoQ had already gone through Phase I trials (rather than mitoTEMPO which is public domain but also showed good results with it in melanoma). FWIW, I found the paper clearer than the EurekaAlert article.
0 -
Research done in Montreal could lead to treatment for [TNBC]
[Using CRISPR to knock out genes systematically, researchers identified two signalling pathways or gene networks of significance in TNBC.]
"One of them was an oncogenic pathway. Those are genes that normally promote cell proliferation on tumours and this one was hyperactivated in those breast cancer patients," Lebrun said.
The second group of genes, which normally act as tumour suppressors by preventing cells to multiply, were found to be inactive or asleep.
This combination, Lebrun believes, could explain why TNBC tumours are so "aggressive and metastatic."
Understanding which genetic mechanisms were involved allowed the team to then uncover existing drugs that targeted those networks.
One of the drugs they tested, Verteporfin, surprisingly had nothing to do with cancer.
"It's actually a drug that is used for disease of the degeneration of the retina. It's an eye disease," Lebrun said... In all, the team evaluated around 10 different drugs, with Verteporfin being one of two standouts... Individually, each [of those two] drug[s] had around a 20 per cent decrease in tumour growth... "But [together] we got something more like 80 per cent," he said. "That's what we call a synergistic effect."
Efforts have been underway since the summer to get Phase 1 clinical trials off the ground... [but] Lebrun said he couldn't give a timeline of when the trial would start and when patients could begin enrolling.
0 -
Warmer Climate Tied to Favorable Breast Cancer Outcomes
Higher environmental temperatures are associated with better outcomes (significant improvements in pCR as well as OS) among patients with stage I-III breast cancer, according to research presented in a poster at the NCCN 2022 Annual Conference.
0 -
onmagosh... debbew.... your post made my day. Is this an excuse to spend the winter in a warm climate? (Access to care issues aside)
 I am such a wimp about the cold and by this time of year, I am ready to petition for no more winter! Now I have a therapeutic excuse. Love it! 0
I am such a wimp about the cold and by this time of year, I am ready to petition for no more winter! Now I have a therapeutic excuse. Love it! 0 -
Lumpie, debbew, & moth:
Thanks for keeping this thread active and informing the community on all the research that's out there. This is such a helpful thread
0 -
Lumpie, I was quite surprised to see that article, too! I wondered if it had anything to do with vitamin D, but that doesn't seem to be what they are thinking.
Norcals, thanks for the nod! Lumpie has led the way but I'm always interested to see what all the other posters have uncovered.
0