Are you currently (or have you been) in a Clinical Trial?
Comments
-
oh, yes, the ATRA in APLeukemia, haven't thought of that in years!! Metformin, its the same reason I wonder about the Ozempic drugs- these metabolic drugs feed into the mTOR/PI3KCA pathway. And also melatonin, the sleep hormone, is an inhibitor of PI3KCA- it pushes cells out of using glucose into mitochondrial ox-phos at night- only MBC cells don't make melatonin and don't do that, they stay using glucose, and they get screwed up if exposed to melatonin.
Reminds me of a paper on circadian rhythm drugs would also be effective against cancer: Nature, 2018 (Panda et al.)
The circadian clock imposes daily rhythms in cell proliferation, metabolism, inflammation and DNA damage response. Perturbations of these processes are hallmarks of cancer and chronic circadian rhythm disruption predisposes individuals to tumour development. This raises the hypothesis that pharmacological modulation of the circadian machinery may be an effective therapeutic strategy for combating cancer. REV-ERBs, the nuclear hormone receptors REV-ERBα (also known as NR1D1) and REV-ERBβ (also known as NR1D2), are essential components of the circadian clock. Here we show that two agonists of REV-ERBs—SR9009 and SR9011—are specifically lethal to cancer cells and oncogene-induced senescent cells, including melanocytic naevi, and have no effect on the viability of normal cells or tissues. The anticancer activity of SR9009 and SR9011 affects a number of oncogenic drivers (such as HRAS, BRAF, PIK3CA and others) and persists in the absence of p53 and under hypoxic conditions. The regulation of autophagy and de novo lipogenesis by SR9009 and SR9011 has a critical role in evoking an apoptotic response in malignant cells. Notably, the selective anticancer properties of these REV-ERB agonists impair glioblastoma growth in vivo and improve survival without causing overt toxicity in mice. These results indicate that pharmacological modulation of circadian regulators is an effective antitumour strategy, identifying a class of anticancer agents with a wide therapeutic window.
https://www.nature.com/articles/nature25170
1 -
However, they have to develop more potent inhibitors of REV-ERB to warrant a clinical trial in humans
0 -
Perky, How are you doing?
A paper came out in Nature Medicine summarizing the phase 1 results of your KAT6 trial.
Inhibition of histone lysine acetyltransferases (KATs) KAT6A and KAT6B has shown antitumor activity in estrogen receptor-positive (ER+) breast cancer preclinical models. PF-07248144 is a selective catalytic inhibitor of KAT6A and KAT6B. In the present study, we report the safety, pharmacokinetics (PK), pharmacodynamics, efficacy and biomarker results from the first-in-human, phase 1 dose escalation and dose expansion study (n = 107) of PF-07248144 monotherapy and fulvestrant combination in heavily pretreated ER+ human epidermal growth factor receptor-negative (HER2−) metastatic breast cancer (mBC). The primary objectives of assessing the safety and tolerability and determining the recommended dose for expansion of PF-07248144, as monotherapy and in combination with fulvestrant, were met. Secondary endpoints included characterization of PK and evaluation of antitumor activity, including objective response rate (ORR) and progression-free survival (PFS). Common treatment-related adverse events (any grade; grades 3–4) included dysgeusia (83.2%, 0%), neutropenia (59.8%, 35.5%) and anemia (48.6%, 13.1%). Exposure was approximately dose proportional. Antitumor activity was observed as monotherapy. For the PF-07248144–fulvestrant combination (n = 43), the ORR (95% confidence interval (CI)) was 30.2% (95% CI = 17.2–46.1%) and the median PFS was 10.7 (5.3–not evaluable) months. PF-07248144 demonstrated a tolerable safety profile and durable antitumor activity in heavily pretreated ER+HER2− mBC. These findings establish KAT6A and KAT6B as druggable cancer targets, provide clinical proof of concept and reveal a potential avenue to treat mBC. clinicaltrial.gov registration: NCT04606446.
2 -
There have been some promising results in the Phase 1/2 trial for a CDK2 inhibitor, BLU-222. When cancers become resistant to CDK4,6i, it is sometimes due to elevated levels of CDK2. Adding a CDK2 inhibitor allows the cell cycle to be shut down more effectively and has been seen to reverse the resistance to CDK4,6i even in some heavily pretreated patients:
3 -
Dear cureious, thanks for very interesting information, especially on CDK2 inhibitor. Through our "carrot-metformin-ozempic" discussion I remembered another clinical trial that uses Alpha-TAE (another form of vitamin E) to tackle/reverse the HER2 resistance: https://clinicaltrials.gov/study/NCT04120246 . I think I read somewhere before that results with mice (again:/) were amazing, hmm, cannot find where that was:/
Saulius
0 -
Thank you for all the info, I will check everything out!
Is the blue-222 available yet? Or just in a trial? And melatonina is anti all breast cancer cell lines or only if you have the PI3KCA?
0 -
Hi, All
I guess I am cross-posting as well. I have recurrent ILC after 16 years and the liver met shows RB1 deletion. It means resistance to the CDK 4/6. I am strong ER+ , PR+ and HER2- negative (low 1). Any trials for RB1 mutations?
0 -
Laguna, Excellent question, one that those of us who have gone through the first lines of endocrine therapy eventually confront when the CDK4,6i and AI/Faslodex finally fail, but we'd still prefer not to be on chemo.
I just now was reading a recent paper reporting that PRMT5 would be a good target for these cancers:
CDK4/6 inhibitors (CDK4/6i) have improved survival of patients with estrogen receptor-positive (ER+) breast cancer. However, patients treated with CDK4/6i eventually develop drug resistance and progress. RB1 loss-of-function alterations confer resistance to CDK4/6i, but the optimal therapy for these patients is unclear. Through a genome-wide CRISPR screen, we identify protein arginine methyltransferase 5 (PRMT5) as a molecular vulnerability in ER+/RB1-knockout breast cancer cells. Inhibition of PRMT5 blocks the G1-to-S transition in the cell cycle independent of RB, leading to growth arrest in RB1-knockout cells. Proteomics analysis uncovers fused in sarcoma (FUS) as a downstream effector of PRMT5. Inhibition of PRMT5 results in dissociation of FUS from RNA polymerase II, leading to hyperphosphorylation of serine 2 in RNA polymerase II, intron retention, and subsequent downregulation of proteins involved in DNA synthesis. Furthermore, treatment with the PRMT5 inhibitor pemrametostat and a selective ER degrader fulvestrant synergistically inhibits growth of ER+/RB-deficient cell-derived and patient-derived xenografts. These findings highlight dual ER and PRMT5 blockade as a potential therapeutic strategy to overcome resistance to CDK4/6i in ER+/RB-deficient breast cancer.
https://www.nature.com/articles/s41467-024-46495-2
1 -
Here is a review. PRMT5 inhibitors exist but have not been tested for ability to restore sensitivity to CDK4,6i. The review also covers other ways that cancer can become resistant to CDK4,6i, including increasing Her2 expression:
0 -
For increased Her2 expression or mutant HER2 cancers (which make Her2 more active):
“Mutations in HER2 happen in approximately 8% of tumors and they do not overlap with other alterations associated with endocrine resistance such as NF1, KRAS, loss of RB1, or FGFR1 amplification. These mutations occur in up to 8% of advanced ER-positive breast cancers, [2%] of primary disease, and approximately 10% to 15% of metastatic invasive lobular carcinomas express these mutations. Most of them are ER-positive and they happen in the absence of HER2 gene amplification. I want to emphasize that these tumors are clinically HER2 negative.”
Common comutations in these cancers include TP53, PIK3CA, ERBB3, and CDH1.1
He also noted that although patients with ER-positive, HER2-mutant breast cancer experienced greater benefit with the combination of fulvestrant and neratinib, acquired secondary HER2 mutations are associated with resistance to neratinib in ER-positive disease.
because rates of pathologic complete response to chemotherapy or endocrine therapy alone are low in invasive lobular breast cancer, investigators plan to combine neratinib and letrozole in an upcoming neoadjuvant trial in patients with HER2-mutant disease.1
0 -
More info from another review:
0 -
This is a more comprehensive review of what comes after CDK4,6i:
1 -
So Laguna, were other actionable targets shown on the genomics panel, like ESR1 or PI3KCA?
0 -
@cure-ious Thank you for the detailed description. Will go through the article as soon as I can. Interesting genetic portrait of mine :)
CARIS: Liver biopsy has BRCA2 and RB1 deletion, but showing AR staining 100%. My HER2 is low (1+). As for PR I am now negative as per pathologist at my hospital even though the report from other place considers it positive. (Strong expression -3, but staining is less than 10%). I even asked my doctor if I am having a prostate cancer as the picture is typical. What’s interesting: I have 23andMe genetic picture from 2014 and it warns me on high probability of prostate cancer based on many genetic pairing combinations and low response to chemo, which leaves me with no options. Funny…I guess I am moving toward triple negative
Guardant360 - liquid biopsy - no mutations whatsoever.I requested GI biopsy genetic testing from CARIS through my MO to see if these mutations are consistent as it’s not necessarily obvious. Still awaiting my ctDNA and complete genetic picture.
Hoping for a miracle drug as only about 3.5% of ILC have RB1 brokenHugs
0 -
Laguna, Can you can take Talazoparib or other PARP inhibitors for the BRCA2 mutation? Also, Enhertu worked even on very low Her2-expressing tumors..Lobular often has high AR staining, so enobosarm (androgen agonist) could be a good option for ER-positive tumors?
0 -
A new type of trial we have not discussed here involves CAR-T immunotherapy directed against MUC1 (aka CA27-29),which has several different trials ongoing. The target is a cleaved part of MUC1 that is often highly expressed in solid tumors (breast, ovarian, colorectal, lung, etc), and not expressed much at all in normal cells. It is just in phase 1, and tho the first breast cancer patient they treated did get a partial response, there are not enough results to know how well it works. This treatment would hit all kinds of cancer cells.
1 -
A recent paper in Nature identifies nerve innervation as a process that accellerates metastasis into organs- and show that this process can be slowed by aprepitant, an anti-nausea medicine (aka Emend).
“Because aprepitant is already approved and safe, oncologists may consider clinical trials to test the impact of this medication on cancer progression in patients with breast cancer,” Tavazoie says"
And of course they will investigate stronger and more selective inhibitors of this process. Here is a lay review:
And the Nature paper:
1 -
The BriaCell IMT technique has worked well for some late-stage patients:
0 -
More info on Bria Cell phase 3 trial:
0 -
@cure-ious I just wanted to say that I read your posts carefully and want to thank you for your time and care and generosity.
3 -
Sorry for necrcposting (meaning, the tread hasn't been active for awhile), but if anyone is still interested in OLEMA I noticed that they are running clinical trials for HER2 treatment. Just scroll down to "clinical trials"
Clinical trials for her2 breast cancer0 -
Artificial Intelligence is moving into the area of MBC biopsies, and promises to be a better and faster predictor in terms of sequencing treatments- new startup in this area:
0 -
Hi Perky,
A question about the sequence of drugs you have been on- you were initially on Ibrance/Faslodex? And now in a trial of KAT6/CDK4/Faslodex? Did your Guardant testing show ESR1 and/or PI3KCA mutations? I ask because many reports saying Faslodex (monotherapy) doesn't work well after progression on Faslodex/CDK4,6i. Yet your experience indicates that the KAT6 drug may reverse the resistance…
0 -
hi, @cure-ious
Just found your post about Blue-222. Will check with my MO on the status of this trial. Release of CDK2 is exactly what happens when CDK4/6 encounters mutated genes like RB or ESR and resistance. But since it is very quiet I guess they have no good data to support the efficacy
Thanks again
0 -
Laguna, I thought I saw something positive recently about BLU-222…They presented at ASCO 2024:
It is clearly still early days, but they say.. "All patients with evaluable ctDNA, a biomarker of tumor burden, treated with the BLU-222 400 mg BID combination dose regimen showed ctDNA reductions. Early evidence of clinical benefit includes an unconfirmed partial response in a patient who had previously progressed following six lines of therapy in the metastatic setting, including prior palbociclib and trastuzumab deruxtecan. These data highlight the impact of CDK2 inhibition when BLU-222 is combined with other therapies."
0 -
Great. I sent them an e-mail requesting any data on RB deleted CDK2 amplification. If they can measure CDK2 in the blood it can be used to see if CDK4/6 is still working or resisted. Based on the article they worked with a specific marker CCNE. Would be interesting to know what they think or ready to try.
Thanks
0 -
Is it typical for a patient themselves to request a clinical trial, or does one normally go through their MO to request evaluation? … will continue looking around for any info on BLU-222
0 -
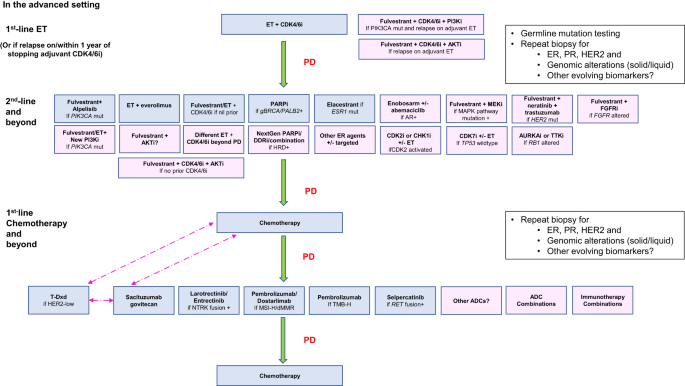
Laguna, This is a good overall review
https://www.sciencedirect.com/science/article/pii/S1040842824000672?via%3Dihub
0 -
0
-
This review covers a new Pfizer CDK4 inhibitor, which is showing promise:
Hence, because of its greater selectivity for CDK4 over CDK6, it leads to less neutropenia in vivo models and, consequently, can be dosed higher to attain tolerated plasma concentrations that exceed those reported for dual CDK4/6i. PF-07220060, either alone or in combination with ET, has been investigated in a multicenter, first-in-human phase 1/2a study (NCT04557449), for advanced solid tumors and HR+/HER2- mBC. At the data cut-off, 26 HR+/HER2- mBC patients (out of 34 with advanced solid tumors) were included. These patients had received escalating doses of PF-07220060 in combination with letrozole or fulvestrant. They were mainly heavily pretreated patients (median number of prior lines: 5 [IQR 1–11]); all had prior CDK4/6i, 19 (73.1%) prior fulvestrant and 20 (76.9%) prior chemotherapy. Most frequent TEAEs with PF-07220060 plus ET were diarrhea (50.0%; 0% G3), neutropenia (50.0%; 15.4% G3), and nausea (38.5%; 3.8% G3), with no G4 TEAEs. A similar safety profile was reported in monotherapy for the other advanced solid tumors. In evaluable mBC cases, the combination of PF-07220060 with ET has demonstrated 6 (28.6%) confirmed responses, including 1 complete response (CR) and 5 partial responses (PR). Clinical benefit response (CR, PR, or ≥24 weeks stable disease) was seen in 11 (52.4%) patients, with a median progression-free survival (PFS) of 24.7 weeks (95% CI: 23.1–47.4). As standalone treatment, 62.1% patients with SD as best response were reported with no partial or complete response and a mPFS of 23,9 wks. At the data cut-off, eight patients (30.8%) continued PF-07220060 + ET without progression for up to 60 plus weeks.
1